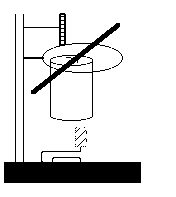
The apparatus should look like this:
Which Fuel Source Has More Heat Energy?
A Comparison Of The Amount Of Energy Given Off In A Combustion Reaction
Objective:
There are two objectives for this experiment. The first is to compare possible fuel sources for the amount of energy they give off in the form of heat energy. The second is to tell which one(s) are cleaner burning fuels than the others.
Review of Scientific Principles:
We are always looking for alternative energy sources since fossil fuels are nonrenewable resources. There are two questions frequently asked when considering an alternative fuel. How much energy will be released per unit of that resource, and what effects might burning it have on the environment? When we use a resource, such as coal, oil, or natural gas to produce energy, we are breaking the chemical bonds within the substance and rearranging them into more stable bonds. This change results in the formation of different products, such as carbon dioxide and water in the case of combustion, and a release of energy. How can we measure the amount of energy?
If we tried to quantify it mechanically, we may never know just how much absolute energy is in the resource itself. Therefore, we use the "heating value" of fuels: how using so much of a certain resource (rearranging its bonds into a more stable state) converts to so much heat (motion of molecules). We all hear every day about counting calories. What is a calorie? A calorie (cal) is defined as the amount of heat needed to raise one gram of water 1o C. A food calorie actually consists of one kilocalorie, or 1,000 calories. Why do we worry about calories in relation to our weight? Energy conservation! If you feed your body more calories than it can use, it will store the energy in a stable state like body fat for you to use and lose later.
For this lab, we will measure the amount of the temperature change and use that to indicate heat energy. Temperature is defined as the average kinetic energy of all the molecules, and heat is the movement of molecules.
Materials and Supplies:
Procedure:
The apparatus should look like this:

Data and Calculations:
| Substance | Initial Temperature oC | Final Temperature oC | Appearance of fumes from burning |
|---|---|---|---|
| Methanol | |||
| Ethanol | |||
| Vegetable oil | |||
| Peanut oil | |||
| Motor oil | |||
| Kerosene |
| Methanol |
|---|
| Ethanol |
| Vegetable oil |
| Peanut oil |
| Motor oil |
| Kerosene |
Experiment 2: Teacher's Notes
Heating it Up!
Which Fuel Source Has More Heat Energy?
Prep Time: 10 minutes
Time: 45-50 minutes
Because of time constraints it works best to 'jigsaw' this lab or have each group do a separate part of this lab; then share their results. Divide the class into six groups (or any multiple of six groups). In order to facilitate this, you'll need to make six clearly labeled 10 ml disposable eye droppers.
Add 10 ml of methanol, ethanol, motor oil, vegetable oil, peanut oil, and kerosene in six different droppers. Label the droppers accordingly. Store upside down in a 50 mL beaker. As you assign each group the material they are to test, point out what table that material is on.
Each group needs to burn the sample they have prepared until the flame extinguishes by itself. (Smoldering samples should be removed and placed in a beaker of water.
Sample Data:
| Substance | Initial Temperature o C | Final Temperature o C | Appearance of fumes from burning |
|---|---|---|---|
| Methanol | 29 | 34 | clean burning |
| Ethanol | 28 | 42 | clean burning |
| Vegetable | 28 | 56 | sooty oil |
| Peanut oil | 28 | 50 | sooty |
| Motor oil | 27 | 55 | sooty |
| Kerosene | 23 | 49 | sooty |
Calculations:
| Methanol | 6 oC |
|---|---|
| Ethanol | 13 oC |
| Vegetable oil | 28 oC |
| Peanut oil | 22 oC |
| Motor oil | 28 oC |
| Kerosene | 26 oC |