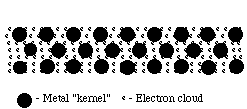
Structure of Metals:
Metals account for about two thirds of all the elements and about 24% of the mass of the planet. They are all around us in such forms as steel structures, copper wires, aluminum foil, and gold jewelry. Metals are widely used because of their properties: strength, ductility, high melting point, thermal and electrical conductivity, and toughness.
These properties also offer clues as to the structure of metals. As with all elements, metals are composed of atoms. The strength of metals suggests that these atoms are held together by strong bonds. These bonds must also allow atoms to move; otherwise how could metals be hammered into sheets or drawn into wires? A reasonable model would be one in which atoms are held together by strong, but delocalized, bonds.
Bonding
Such bonds could be formed between metal atoms that have low electronegativities and do not attract their valence electrons strongly. This would allow the outermost electrons to be shared by all the surrounding atoms, resulting in positive ions (cations) surrounded by a sea of electrons (sometimes referred to as an electron cloud).

Because these valence electrons are shared by all the atoms, they are not considered to be associated with any one atom. This is very different from ionic or covalent bonds, where electrons are held by one or two atoms. The metallic bond is therefore strong and uniform. Since electrons are attracted to many atoms, they have considerable mobility that allows for the good heat and electrical conductivity seen in metals.
Above their melting point, metals are liquids, and their atoms are randomly arranged and relatively free to move. However, when cooled below their melting point, metals rearrange to form ordered, crystalline structures.
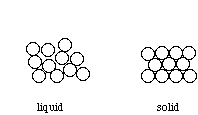
Crystals
To form the strongest metallic bonds, metals are packed together as closely as possible. Several packing arrangements are possible. Instead of atoms, imagine marbles that need to be packed in a box. The marbles would be placed on the bottom of the box in neat orderly rows and then a second layer begun. The second layer of marbles cannot be placed directly on top of the other marbles and so the rows of marbles in this layer move into the spaces between marbles in the first layer. The first layer of marbles can be designated as A and the second layer as B giving the two layers a designation of AB.
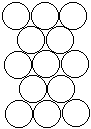 | 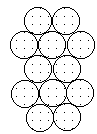 | 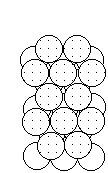 |
|---|---|---|
| Layer "A" | Layer "B" | AB packing |
Packing marbles in the third layer requires a decision. Again rows of atoms will nest in the hollows between atoms in the second layer but two possibilities exist. If the rows of marbles are packed so they are directly over the first layer (A) then the arrangement could be described as ABA. Such a packing arrangement with alternating layers would be designated as ABABAB. This ABAB arrangement is called hexagonal close packing (HCP).
If the rows of atoms are packed in this third layer so that they do not lie over atoms in either the A or B layer, then the third layer is called C. This packing sequence would be designated ABCABC, and is also known as face-centered cubic (FCC). Both arrangements give the closest possible packing of spheres leaving only about a fourth of the available space empty.
The smallest repeating array of atoms in a crystal is called a unit cell. A third common packing arrangement in metals, the body-centered cubic (BCC) unit cell has atoms at each of the eight corners of a cube plus one atom in the center of the cube. Because each of the corner atoms is the corner of another cube, the corner atoms in each unit cell will be shared among eight unit cells. The BCC unit cell consists of a net total of two atoms, the one in the center and eight eighths from the corners.
In the FCC arrangement, again there are eight atoms at corners of the unit cell and one atom centered in each of the faces. The atom in the face is shared with the adjacent cell. FCC unit cells consist of four atoms, eight eighths at the corners and six halves in the faces. Table 1 shows the stable room temperature crystal structures for several elemental metals.
| Aluminum | FCC | Nickel | FCC | |
|---|---|---|---|---|
| Cadmium | HCP | Niobium | BCC | |
| Chromium | BCC | Platinum | FCC | |
| Cobalt | HCP | Silver | FCC | |
| Copper | FCC | Titanium | HCP | |
| Gold | FCC | Vanadium | BCC | |
| Iron | BCC | Zinc | HCP | |
| Lead | FCC | Zirconium | HCP | |
| Magnesium | HCP |
Unit cell structures determine some of the properties of metals. For example, FCC structures are more likely to be ductile than BCC, (body centered cubic) or HCP (hexagonal close packed). Figure 4 shows the FCC and BCC unit cells. (See Crystal Structure Activity)
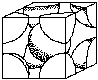 | 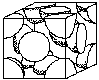 |
|---|---|
| Body Centered Cubic | Face Centered Cubic |
As atoms of melted metal begin to pack together to form a crystal lattice at the freezing point, groups of these atoms form tiny crystals. These tiny crystals increase in size by the progressive addition of atoms. The resulting solid is not one crystal but actually many smaller crystals, called grains. These grains grow until they impinge upon adjacent growing crystals. The interface formed between them is called a grain boundary. Grains are sometimes large enough to be visible under an ordinary light microscope or even to the unaided eye. The spangles that are seen on newly galvanized metals are grains. (See A Particle Model of Metals Activity) Figure 5 shows a typical view of a metal surface with many grains, or crystals.

Crystal Defects:
Metallic crystals are not perfect. Sometimes there are empty spaces called vacancies, where an atom is missing. Another common defect in metals are dislocations, which are lines of defective bonding. Figure 6 shows one type of dislocation.
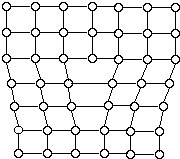
These and other imperfections, as well as the existence of grains and grain boundaries, determine many of the mechanical properties of metals. When a stress is applied to a metal, dislocations are generated and move, allowing the metal to deform.
Mechanical Properties:
When small loads (stresses) are applied to metals they deform, and they return to their original shape when the load is released. Bending a sheet of steel is an example where the bonds are bent or stretched only a small percentage. This is called elastic deformation and involves temporary stretching or bending of bonds between atoms.
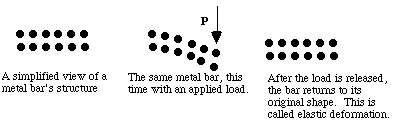
When higher stresses are applied, permanent (plastic) deformation occurs. For example, when a paper clip is bent a large amount and then released, it will remain partially bent. This plastic deformation involves the breaking of bonds, often by the motion of dislocations. See Figure 8. Dislocations move easily in metals, due to the delocalized bonding, but do not move easily in ceramics. This largely explains why metals are ductile, while ceramics are brittle.

If placed under too large of a stress, metals will mechanically fail, or fracture. This can also result over time from many small stresses. The most common reason (about 80%) for metal failure is fatigue. Through the application and release of small stresses (as many as millions of times) as the metal is used, small cracks in the metal are formed and grow slowly. Eventually the metal is permanently deformed or it breaks (fractures). (See Processing Metals Activity)
Processing:
In industry, molten metal is cooled to form the solid. The solid metal is then mechanically shaped to form a particular product. How these steps are carried out is very important because heat and plastic deformation can strongly affect the mechanical properties of a metal.
Grain Size Effect:
It has long been known that the properties of some metals could be changed by heat treating. Grains in metals tend to grow larger as the metal is heated. A grain can grow larger by atoms migrating from another grain that may eventually disappear. Dislocations cannot cross grain boundaries easily, so the size of grains determines how easily the dislocations can move. As expected, metals with small grains are stronger but they are less ductile. Figure 5 shows an example of the grain structure of metals.
Quenching and Hardening:
There are many ways in which metals can be heat treated. Annealing is a softening process in which metals are heated and then allowed to cool slowly. Most steels may be hardened by heating and quenching (cooling rapidly). This process was used quite early in the history of processing steel. In fact, it was believed that biological fluids made the best quenching liquids and urine was sometimes used. In some ancient civilizations, the red hot sword blades were sometimes plunged into the bodies of hapless prisoners! Today metals are quenched in water or oil. Actually, quenching in salt water solutions is faster, so the ancients were not entirely wrong.
Quenching results in a metal that is very hard but also brittle. Gently heating a hardened metal and allowing it to cool slowly will produce a metal that is still hard but also less brittle. This process is known as tempering. (See Processing Metals Activity). It results in many small Fe3C precipitates in the steel, which block dislocation motion which thereby provide the strengthening.
Cold Working:
Because plastic deformation results from the movement of dislocations, metals can be strengthened by preventing this motion. When a metal is bent or shaped, dislocations are generated and move. As the number of dislocations in the crystal increases, they will get tangled or pinned and will not be able to move. This will strengthen the metal, making it harder to deform. This process is known as cold working. At higher temperatures the dislocations can rearrange, so little strengthening occurs.
You can try this with a paper clip. Unbend the paper clip and bend one of the straight sections back and forth several times. Imagine what is occurring on the atomic level. Notice that it is more difficult to bend the metal at the same place. Dislocations have formed and become tangled, increasing the strength. The paper clip will eventually break at the bend. Cold working obviously only works to a certain extent! Too much deformation results in a tangle of dislocations that are unable to move, so the metal breaks instead.
Heating removes the effects of cold-working. When cold worked metals are heated, recrystallization occurs. New grains form and grow to consume the cold worked portion. The new grains have fewer dislocations and the original properties are restored.
Alloys:
The presence of other elements in the metal can also change its properties, sometimes drastically. The arrangement and kind of bonding in metals permits the addition of other elements into the structure, forming mixtures of metals called alloys. Even if the added elements are nonmetals, alloys may still have metallic properties.
Copper alloys were produced very early in our history. Bronze, an alloy of copper and tin, was the first alloy known. It was easy to produce by simply adding tin to molten copper. Tools and weapons made of this alloy were stronger than pure copper ones. Adding zinc to copper produces another alloy, brass. Although brass is more difficult to produce than bronze, it also was known in ancient times. (See "Gold" Penny Activity) Typical composition of some alloys is given in Table 2.
| Alloy | Composition |
|---|---|
| Brass | Copper, Zinc |
| Bronze | Copper, Zinc, Tin |
| Pewter | Tin, Copper, Bismuth, Antimony |
| Solder | Lead, Tin |
| Alnico | Aluminum, Nickel, Cobalt, Iron |
| Cast iron | Iron, Carbon, Manganese, Silicon |
| Steel | Iron, Carbon (plus small amounts of alloying elements) |
| Stainless Steel | Iron, Chromium, Nickel |
Alloys are mixtures and their percentage composition can vary. This is useful, because the properties of alloys can be manipulated by varying composition. For example, electricians need a solder with different properties than the one used by plumbers. Electrical solder hardens very quickly producing an almost immediate connection. This would not be practical for plumbers who need some time to set the joint. Electrical solder contains about 60% tin, whereas plumber's solder contains about 30%.
Pewter originally contained lead, and since pewter was used for plates and goblets, it probably was a source of lead poisoning. Pewter made today is lead-free. Increased knowledge of the properties of metals also leads to new alloys. Some brasses form shape memory alloys which can be bent and will return to their original shape when gently heated. Zinc alloys, used as a coating on steel, slow corrosion (galvanized steel). Cadmium alloys find extensive use in solar cells. The ability of cupronickel to resist the build-up of deposits makes it useful for cages in fish farming.
Iron and Steel:
Carbon steels vary in the percentage of carbon they contain. The amount of carbon affects the properties of the steel and its suitability for specific uses. Steels rarely contain more than 1% carbon. Structural steel contains about 0.1-0.2% carbon by weight; this makes it slightly more ductile and less apt to break during earthquakes. Steel used for tools is about 0.5-1 % carbon, making it harder and more wear resistant. Cast iron is between 2.5 and 4% carbon and finds use in low cost applications where its brittleness is not a problem. Surprisingly, pure iron is extremely soft and is rarely used. Increasing the amount of carbon tends to increase the hardness of the metal as shown by the following graph. In slowly cooled steels, carbon increases the amount of hard Fe3C; in quenched steels, it also increases the hardness and strength of the material.
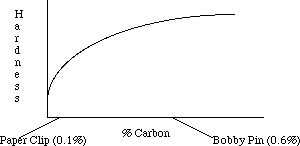
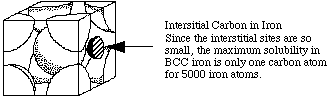
Bobby pins and paper clips are processed in much the same way but contain different amounts of carbon. Bobby pins and paper clips are formed from cold worked steel wire. The paper clip, containing little carbon, is mostly pure Fe with some Fe3C particles. The bobby pin has more carbon and thus contains a larger amount of Fe3C which makes it much harder and stronger.
The properties of steel can be tailored for special uses by the addition of other metals to the alloy. Titanium, vanadium, molybdenum and manganese are among the metals added to these specialty steels. Stainless steel contains a minimum of 12% chromium, which stops further oxidation by forming a protective oxide on the surface.
Corrosion:
Corrosion of metals can be a major problem, especially for long-term structural applications like cars, bridges, and ships. Most corrosion is electrochemical (galvanic) in nature. To have corrosion, an anode (a more easily oxidized region) and a cathode (a less easily oxidized region) must be present. These may be different types of metals or simply different regions on the same metal. Some sort of electrolyte that can allow the transport of electrons must also be present. Corrosion involves the release of electrons at the anode due to the high oxidation potential of the atoms at the anode. As the electrons are released, metal cations are formed and the metal disintegrates. Simultaneously, the cathode, which has a greater reduction potential, accepts the electrons by either forming negative ions or neutralizing positive ions.
In the case of the activity or electromotive force series, a metal such as zinc reacts with hydrogen and serves as both the anode and the cathode. (See Activity Series Activity) The equation for this reaction is:
Hydrogen bubbles at the cathode while the anode is destroyed. Surface imperfections, the presence of impurities, orientation of the grains, localized stresses, and variations in the environment are some of the factors determining why a single piece of metal may serve as both electrodes. For example, the head and point of a nail have been cold worked and can serve as the anode while the body serves as the cathode. (See Corrosion of Iron Activity)
Although oxidation at the anode and reduction at the cathode are simultaneous processes, corrosion usually occurs at the anode. The cathode is almost never destroyed. In 1824, Davy developed a method of protecting the hulls of ships from corrosion by using zinc that can be periodically replaced. Zinc is more active than the steel in the hull and will serve as the anode and be corroded; it is sacrificed to protect the steel structure. The steel that would have been both the anode and cathode normally serves as the cathode. This is called cathodic protection. Pipe lines are similarly protected by the more active metal magnesium. Sometimes electric currents are maintained in short sections of pipe lines with a length of similar metal wired to serve as the sacrificial anode.
Corrosion is a major problem that must be solved in order to effectively utilize metals. Iron combines with oxygen in the air forming iron oxide (rust), eventually destroying the usefulness of the metal. (See Optional: Chemical Hand Warmer Activity) Fortunately, some metals, such as aluminum and chromium, form a protective oxide coating that prevents further oxidation (corrosion). Similarly, copper combines with sulfur and oxygen forming the familiar green patina.
Understanding the chemistry of metals leads to the development of methods to reduce and prevent corrosion. Chromium atoms are about the same size as iron atoms and can substitute for them in iron crystals. Chromium forms an oxide layer that allows stainless steel to resist corrosion. Metals can be painted or they can be coated with other metals; galvanized (zinc coated) steel is an example. When these two metals are used together, the more active zinc corrodes, sacrificing itself to save the steel.
Metal Ores:
Gold, silver, and copper were the first metals used because they are found in the free or elemental state. Most metals found in nature are combined with other elements such as oxygen and sulfur. Energy is needed to extract metals from these compounds or ores. Historically, the ease with which a given metal could be extracted from its ore, along with availability, determined when it came into use, hence the early use of copper, tin, and iron. The formulas for some ores are given below:
| Hematite | Fe2O3 | Rutile | TiO2 |
|---|---|---|---|
| Magnetite | Fe3O4 | Zircon | ZrSiO4 |
| Pyrite | FeS2 | Cassiterite | SnO2 |
| Chalcocite | Cu2S | Bauxite | Al2O3 |
| Cinnabar | HgS | Galena | PbS |
These ores are ionic compounds in which the metals exist as positive ions. For example the oxidation state of iron in hematite is +3; the oxidation state of copper in chalcocite is +1. Extracting metals from their ores is an oxidation-reduction (Redox) reaction. In the elemental state, metals consist of atoms not ions. Since atoms have no overall charge the metal ions gain electrons in the reaction; they are reduced.
The overall reaction for the reduction of copper from chalcocite is:
This is the overall reaction only. The complete process is not this simple. The reduction of metals from their ores typically requires a series of chemical and mechanical processes. These are usually energetically expensive, consuming large amounts of heat and/or electrical energy. For example, about five percent of the electricity consumed in the United States is used to produce aluminum. It costs about one hundred times as much to make an aluminum pop can, starting with the ore, as it does to melt and form recycled aluminum. Extracting metals from ores may also produce pollutants such as the sulfur dioxide above. Whenever possible, recycling and reprocessing metals makes sense.
The relative difficulty of extracting metals from their ores indicates that this is their preferred state. Once removed from their ores, and in the elemental state, most metals display considerable tendency to react with oxygen and sulfur and return to their natural state; they corrode! In corrosion, the metal is oxidized. It loses electrons, becoming a positive ion. (See Corrosion of Metals Activity)
Metals Summary
Metals have useful properties including strength, ductility, high melting points, thermal and electrical conductivity, and toughness. They are widely used for structural and electrical applications. Understanding the structure of metals can help us understand their properties.
Metal atoms are attached to each other by strong, delocalized bonds. These bonds are formed by a cloud of valence electrons that are shared between positive metal ions (cations) in a crystal lattice. In this arrangement, the valence electrons have considerable mobility and are able to conduct heat and electricity easily. In the crystal lattice, metal atoms are packed closely together to maximize the strength of the bonds. An actual piece of metal consists of many tiny crystals called grains that touch at grain boundaries.
Due to the delocalized nature of the bonds, metal atoms are able to slide past each other when the metal is deformed instead of fracturing like a brittle material. This movement of atoms is accomplished through the generation and movement of dislocations in the lattice. Processing techniques that change the bonding between atoms or affect the number or mobility of dislocations can have a large effect on the mechanical properties of a metal.
Elastic deformation of a metal is a small change in shape at low stress which is recoverable after the stress is removed. This type of deformation involves stretching of the metal bonds, but the atoms do not slide past each other. Plastic deformation occurs when the stress is sufficient to permanently deform the metal. This type of deformation involves the breaking of bonds, usually by the movement of dislocations.
Plastic deformation results in the formation of more dislocations in the metal lattice. This can result in a decrease in the mobility of these dislocations due to their tendency to become tangled or pinned. Plastic deformation at temperatures low enough that atoms cannot rearrange (cold-working), can strengthen a metal as a result of this effect. One side effect is that the metal becomes more brittle. As a metal is used, cracks tend to form and grow, eventually causing it to break or fracture.
Dislocations cannot easily cross grain boundaries. If a metal is heated, the grains can grow larger and the material becomes softer. Heating a metal and cooling it quickly (quenching), followed by gentle heating (tempering), results in a harder material due to the formation of many small Fe3C precipitates which block dislocations.
Mixing of metals with other metals or nonmetals can result in alloys that have desirable properties. Steel formed from iron and carbon can vary substantially in hardness depending on the amount of carbon added and the way in which it was processed. Some alloys have a higher resistance to corrosion.
Corrosion is a major problem with most metals. It is an oxidation-reduction reaction in which metal atoms form ions causing the metal to weaken. One technique that has been developed to combat corrosion in structural applications includes the attachment of a sacrificial anode made of a metal with a higher oxidation potential. In this arrangement, the anode corrodes, leaving the cathode, the structural part, undamaged. The formation of a protective coating on the outside of a metal can also resist corrosion. Steels that contain chromium metal form a protective coating of chromium oxide. Aluminum is also corrosion resistant due to the formation of a strong oxide coating. Copper forms the familiar green patina by reacting with sulfur and oxygen in the air.
Only a few pure metals can be found in nature. Most metals exist as ores, compounds of the metal with oxygen or sulfur. Separating the pure metal from the ore often involves large amounts of energy as heat and/or electricity. Due to this large expenditure of energy, it makes sense to recycle metals when possible.
Discussion Questions
1. How are ores extracted from the earth?
2. Name 4 alloys and the metals from which they are made.
3. What impact does "cold working" have on metals?
4. What process makes metals hard, but brittle?
5. What process makes metals softer and easier to work?
6. Give three methods used to reduce corrosion.
7. Give 2 valuable impacts of recycling.
Problem
Assume the radius of one iron atom is 1.24 angstroms (1 angstrom = 1 x 10-8 cm). What would be the density of body centered cubic (BCC) iron in grams/cubic centimeter? Hint: Find the mass and volume of one unit cell. Remember to count only the fraction of each atom in the cell.
Extension:
The maximum solubility of carbon in BCC iron is one atom for every 5000 atoms of iron. What would be the density of steel with the maximum amount of carbon dissolved?
Solution
![]() = m/V = # atoms x (mass/atom) / cell volume
= m/V = # atoms x (mass/atom) / cell volume
In BCC iron, there are two iron atoms per unit cell. (8 x 1/8 + 1)
One iron atom has a mass of 55.85 amu or 9.27 x 10-23 grams.
The total mass of one unit cell is 1.85 x 10-22 grams.
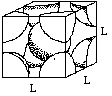
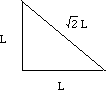
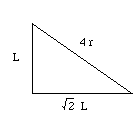
Let (r) be the radius of an iron atom. The atoms at the corners contact the atom in the middle, making the diagonal of the box equal to (4r).
If we call one side of the box (L), a diagonal of the cube face would be equal to (square root of 2) times (L).
One side, the diagonal of the cube face, and the diagonal of the box make a right triangle. Using the Pythagorean theorem, (L)2 + (square root 2 x (L))2 = (4r)2.
Solving for L and plugging in for (r), we find that L = 2.86 angstroms or 2.86 x 10-8 cm.
The volume of the cube (unit cell) is (L)3 = 2.34 x 10-23 cm3. Dividing the mass by the volume we get :
Density = 7.91 grams/cm3.