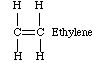
The field of polymers is so vast and the applications so varied, that it is important to understand how polymers are made and used. Since there are over 60,000 different plastics vying for a place in the market, knowledge of this important field can truly enrich our appreciation of this wonder material. Companies manufacture over 30 million tons of plastics each year, and spend large sums on research, development, and more efficient recycling methods. Below we learn some of the scientific principles involved in the production and processing of these fossil fuel derived materials known as polymers.
Polymerization Reactions
The chemical reaction in which high molecular mass molecules are formed from monomers is known as polymerization. There are two basic types of polymerization, chain-reaction (or addition) and step-reaction (or condensation) polymerization.
Chain-Reaction Polymerization
One of the most common types of polymer reactions is chain-reaction (addition) polymerization. This type of polymerization is a three step process involving two chemical entities. The first, known simply as a monomer, can be regarded as one link in a polymer chain. It initially exists as simple units. In nearly all cases, the monomers have at least one carbon-carbon double bond. Ethylene is one example of a monomer used to make a common polymer.
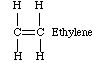
Step 1: Initiation
The first step in the chain-reaction polymerization process, initiation, occurs when the free-radical catalyst reacts with a double bonded carbon monomer, beginning the polymer chain. The double carbon bond breaks apart, the monomer bonds to the free radical, and the free electron is transferred to the outside carbon atom in this reaction.
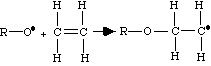
Step 2: Propagation
The next step in the process, propagation, is a repetitive operation in which the physical chain of the polymer is formed. The double bond of successive monomers is opened up when the monomer is reacted to the reactive polymer chain. The free electron is successively passed down the line of the chain to the outside carbon atom.
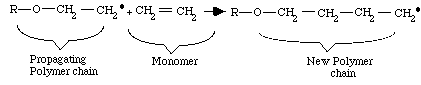
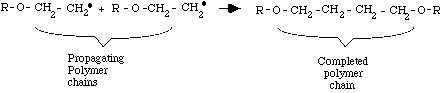
Step 3: Termination
Termination occurs when another free radical (R-O.), left over from the original splitting of the organic peroxide, meets the end of the growing chain. This free-radical terminates the chain by linking with the last CH2.
component of the polymer chain. This reaction produces a complete polymer chain. Termination can also occur when two unfinished chains bond together. Both termination types are diagrammed below. Other types of termination are also possible.
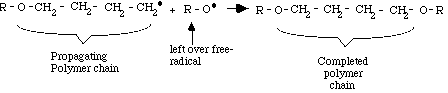
Step-Reaction Polymerization
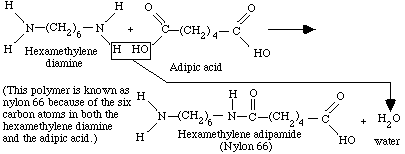
Step-reaction (condensation) polymerization is another common type of polymerization. This polymerization method typically produces polymers of lower molecular weight than chain reactions and requires higher temperatures to occur. Unlike addition polymerization, step-wise reactions involve two different types of di-functional monomers or end groups that react with one another, forming a chain. Condensation polymerization also produces a small molecular by-product (water, HCl, etc.). Below is an example of the formation of Nylon 66, a common polymeric clothing material, involving one each of two monomers, hexamethylene diamine and adipic acid, reacting to form a
dimer of Nylon 66.
Polymer Chemical Structure
The monomers in a polymer can be arranged in a number of different ways. As indicated above, both addition and condensation polymers can be linear, branched, or cross-linked. Linear polymers are made up of one long continuous chain, without any excess appendages or attachments. Branched polymers have a chain structure that consists of one main chain of molecules with smaller molecular chains branching from it. A branched chain-structure tends to lower the degree of crystallinity and density of a polymer. Cross-linking in polymers occurs when primary valence bonds are formed between separate polymer chain molecules.
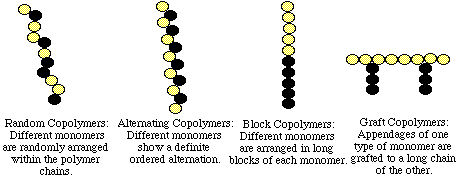
Chains with only one type of monomer are known as
homopolymers. If two or more different type monomers are involved, the resulting copolymer can have several
configurations or arrangements of the monomers along the chain. The four main configurations are depicted below:
Polymer Physical Structure
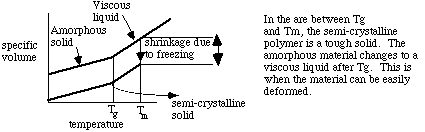
Segments of polymer molecules can exist in two distinct physical structures. They can be found in either crystalline or amorphous forms. Crystalline polymers are only possible if there is a regular chemical structure (e.g., homopolymers or alternating copolymers), and the chains possess a highly ordered arrangement of their segments. Crystallinity in polymers is favored in symmetrical polymer chains, however, it is never 100%. These semi-crystalline polymers possess a rather typical liquefaction pathway, retaining their solid state until they reach their melting point at Tm.
Amorphous polymers do not show order. The molecular segments in amorphous polymers or the amorphous domains of semi-crystalline polymers are randomly arranged and entangled. Amorphous polymers do not have a definable
Tm due to their randomness. At low temperatures, below their glass transition temperature (Tg), the segments are immobile and the sample is often brittle. As temperatures increase close to Tg, the molecular segments can begin to move. Above Tg, the mobility is sufficient (if no crystals are present) that the polymer can flow as a highly viscous liquid. The viscosity
decreases with increasing temperature and decreasing molecular weight. There can also be an elastic response if the entanglements cannot align at the rate a force is applied (as in silly putty). This material is then described as visco-elastic. In a semi-crystalline polymer, molecular flow is prevented by the portions of the molecules in the crystals until the temperature is above Tm. At this point a visco-elastic material forms. These effects can most easily be seen on a specific volume versus temperature graph.
Members of the Polymer Family
Polymers can be separated into two different groups depending on their behavior when heated. Polymers with linear molecules are likely to be thermoplastic. These are substances that soften upon heating and can be remolded and recycled. They can be semi-crystalline or amorphous. The other group of polymers is known as thermosets. These are substances that do not soften under heat and pressure and cannot be remolded or recycled. They must be remachined, used as fillers, or incinerated to remove them from the environment.
Thermoplastics
Thermoplastics are generally carbon containing polymers synthesized by addition or condensation polymerization. This process forms strong covalent bonds within the chains and weaker secondary Van der Waals bonds between the chains. Usually, these secondary forces can be easily overcome by thermal energy, making thermoplastics moldable at high temperatures. Thermoplastics will also retain their newly reformed shape after cooling. A few common applications of thermoplastics include: parts for common household appliances, bottles, cable insulators, tape, blender and mixer bowls, medical syringes, mugs, textiles, packaging, and insulation.
Thermosets
Thermosets have the same Van der Waals bonds that thermoplastics do. They also have a stronger linkage to other chains. Strong covalent bonds chemically hold different chains together in a thermoset material. The chains may be directly bonded to each other or be bonded through other molecules. This "cross-linking" between the chains allows the material to resist softening upon heating. Thus, thermosets must be machined into a new shape if they are to be reused or they can serve as powdered fillers. Although thermosets are difficult to reform, they have many distinct advantages in engineering design applications including:
A few common applications for thermosets include epoxies (glues), automobile body parts, adhesives for plywood and particle board, and as a matrix for composites in boat hulls and tanks.
Polymer Processing
There are five basic processes to form polymer products or parts. These include; injection molding, compression molding, transfer molding, blow molding, and extrusion. Compression molding and transfer molding are used mainly for thermosetting plastics. Injection molding, extrusion and blow molding are used primarily with thermoplastics.
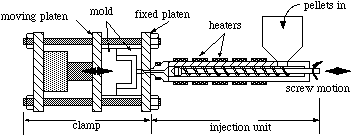
Injection Molding
This very common process for forming plastics involves four steps:
Advantages of injection molding include rapid processing, little waste, and easy automation. Molded parts include combs, toothbrush bases, pails, pipe fittings, and model airplane parts.
Compression Molding
This type of molding was among the first to be used to form plastics. It involves four steps:
For thermoplastics, the mold is cooled before removal so the part will not lose its shape. Thermosets may be ejected while they are hot and after curing is complete. This process is slow, but the material moves only a short distance to the mold, and does not flow through gates or runners. Only one part is made from each mold.
Transfer Molding
This process is a modification of compression molding. It is used primarily to produce thermosetting plastics. Its steps are:
Mold costs are expensive and much scrap material collects in the sprues and runners, but complex parts of varying thickness can be accurately produced.
Blow Molding
Blow molding produces bottles, globe light fixtures, tubs, automobile gasoline tanks, and drums. It involves:
This process is rapid and relatively inexpensive.
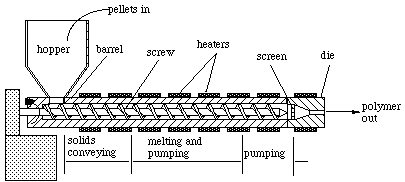
Extrusion
This process makes parts of constant cross section like pipes and rods. Molten polymer goes through a die to produce a final shape. It involves four steps:
An extruder has a hopper to feed the polymer and additives, a barrel with a continuous feed screw, a heating element, and a die holder. An adapter at the end of an extruder blowing air through an orifice into the hot polymer extruded through a ring die produces plastic bags and films.
| Process | Thermoplastic (TP) or Thermoset (TS) | Advantages | Disadvantages |
|---|---|---|---|
| Injection Molding | TP, TS | It has the most precise control of shape and dimensions, is a highly automatic process, has fast cycle time, and the widest choice of materials. | It has high capital cost, is only good for large numbers of parts, and has large pressures in mold (20,000 psi). |
| Compression Molding | TS | It has lower mold pressures (1000 psi), does minimum damage to reinforcing fibers (in composites), and large parts are possible. | It requires more labor, longer cycle than injection molding, has less shape flexibility than injection molding, and each charge is loaded by hand. |
| Transfer Molding | TS | It is good for encapsulating metal parts and electronic circuits. | There is some scrap with every part and each charge is loaded by hand. |
| Blow Molding | TP | It can make hollow parts (especially bottles), stretching action improves mechanical properties, has a fast cycle, and is low labor. | It has no direct control over wall thickness, cannot mold small details with high precision, and requires a polymer with high melt strength. |
| Extrusion | TP | It is used for films, wraps, or long continuos parts (ie. pipes). | It must be cooled below its glass transition temperature to maintain stability. |
Recycling: Today's Challenge, Tomorrow's Reward
Overview
Consumer waste in the United States poses a challenge to everyone. Waste solid materials can be grouped into the following categories:
Today, consumers are using more products and, therefore, producing more solid waste. As time goes by, we find ourselves with less space to put this waste. Eighty percent of all solid waste is buried in landfills. Today there are one third fewer landfills in operation than the 18,500 available a decade ago, making land-filling much more expensive.
Tipping fees, the charge to the waste hauler for dumping a load of solid waste, have been increasing regularly. Municipalities have imposed restrictions and/or have banned the startup of new landfills within their boundaries. As an example, 50% of New Jersey's solid waste is shipped out of state for landfill burial.
The amount which synthetic polymers contribute to the weight of solid waste will continue to go up as the use of plastics increases as projected below.
| Year | Total Waste | Percentage Plastics |
|---|---|---|
| 1960 | 76 million tons | 2.7% |
| 1984 | 133 million tons | 7.2% |
| 1995 | 142 million tons | 8.4% |
| 2000 | 159 million tons | 9.8% (projected) |
Plastics constitute between 14 and 22% of the volume of solid waste. One possible answer to this problem is recycling. In 1990, 1 to 2% of plastics, 29% of aluminum, 25% of paper, 7% of glass, and 3% of rubber and steel as post consumer wastes were recycled. Obviously, increasing the amount of plastics recycled would appear to be the answer. However, a major handicap in the reuse of plastics is that reprocessing adds a heat history, degrades properties and makes repeat use for the same application difficult. For example, the 58 gram, 2-liter polyethylene terephthalate (PET) beverage bottle consists of 48 g of PET, the rest being a high density polyethylene (HDPE) cup base, paper label, adhesive, and molded polypropylene (PP) cap. The cup base, label, adhesive and cap are contaminants in the recycling of the PET.
In response to the contaminants issue in plastic recycling, plastic products are being designed "reuse-friendly". Products are being made with recyclability as a viable means for disposal. At least one company has designed a 2-liter beverage bottle made of all PET for cost effective recycling. Concerning the reuse of recycled plastics, many organizations are reevaluating the use of recycled plastics. As an example, plastic beads are being used to remove paint from aircraft employing a "sand blasting" type method. Previously, harsh, environmentally unfriendly chemical solvents were used. The use of recycled plastics is only limited by the imagination of the designers and end users of the plastics.
Another reason for not discarding plastics is the conservation of energy. The energy value of polyethylene (PE) is 100 % of an equivalent mass of #2 heating oil. Polystyrene (PS) is 75%, while polyvinyl chloride (PVC) and PET are about 50%. With the energy value of a pound of #2 heating oil at 20,000 B.T.U., land filling plastics results in a waste of energy. Some countries, notably Japan, tap into the energy value of plastic and paper with waste-to-energy incinerators.
Another factor in the recycling equation is the economic trend of increasing tipping fees at landfills. In northeastern states, tipping fees have progressively increased, but in western states the fees have remained low due to the local government subsidies to landfills. As the cost of land filling of solid waste increases, so does the incentive to recycle. When the cost of land filling exceeds the cost of recycling, recycling will be a practical alternative to land filling.
These factors have led to certain recommendations by the United States Environmental Protection Agency. In order of highest to lowest, the EPA's recommendations are: source reduction, recycling, thermal reduction (incineration), and land filling. Each of these is not without its problems. Source reduction calls for the redesigning of packaging and/or the use of less, lighter, or more environmentally safe materials. The trade-off could mean reduced food packaging with the possibility of higher food spoilage rates. There would be fewer plastics, but more food in solid waste to be disposed. Whatever disposal method is chosen, the choice is complex. Whatever the costs, the consumer will bear them.
Recycling of Different Plastics
PET (polyethylene terephthalate)
In 1989, a billion pounds of virgin PET were used to make beverage bottles of which about 20% was recycled. Of the amount recycled, 50% was used for fiberfill and strapping. The reprocessors claim to make a high quality, 99% pure, granulated PET. It sells at 35 to 60% of virgin PET costs.
The major reuses of PET include sheet, fiber, film, and extrusions. When chemically treated, the recycled product can be converted into raw materials for the production of unsaturated polyester resins. If sufficient energy is used, the recycled product can be depolymerized to ethylene glycol and terephthalic acid and then repolymerized to virgin PET.
HDPE (high density polyethylene)
Of the plastics that have a potential for recycling, the rigid HDPE container is the one most likely to be found in a landfill. Less than 5% of HDPE containers are treated or processed in a manner that makes recycling easy. Virgin HDPE is used in opaque household and industrial containers used to package motor oil, detergent, milk, bleach, and agricultural chemicals.
There is a great potential for the use of recycled HDPE in base cups, drainage pipes, flower pots, plastic lumber, trash cans, automotive mud flaps, kitchen drain boards, beverage bottle crates, and pallets. Most recycled HDPE is a colored opaque material, that is available in a multitude of tints.
LDPE (low density polyethylene)
LDPE is recycled by giant resin suppliers and merchant processors either by burning it as a fuel for energy or reusing it in trash bags. Recycling trash bags is a big business. Their color is not critical, therefore, regrinds go into black, brown, and to some lesser extent, green and yellow bags.
PVC (polyvinyl chloride)
There is much controversy concerning the recycling and reuse of PVC due to health and safety issues. When PVC is burned, the effects on the incinerator and quality of the air are often questioned. The Federal Food and Drug Administration (FDA) has ordered its staff to prepare environmental impact statements covering PVC's role in landfills and incineration. The burning of PVC releases toxic dioxins, furans, and hydrogen chloride. These fumes are carcinogenic, mutagenic, and teratagenic. This is one of the reasons why PVC must be identified and removed from any plastic waste to be recycled.
Currently, PVC is used in food and alcoholic beverage containers with FDA approval. The future of PVC rests in the hands of the plastics industry to resolve the issue of the toxic effects of the incineration of PVC. It is of interest to note that PVC accounts for less than 1% of land fill waste. When PVC is properly recycled, the problems of toxic emissions are minimized. Various recyclers have been able to reclaim PVC without the health problems. Uses for recycled PVC include aquarium tubing, drainage pipe, pipe fittings, floor tile, and nonfood bottles. When PVC is combined with other plastic waste it has been used to produce plastic lumber.
PS (polystyrene)
PS and its manufacturers have been the target of environmentalists for several years. The manufacturers and recyclers are working hard to make recycling of PS as common as that of paper and metals. One company, Rubbermaid, is testing reclaimed PS in service trays and other utility items. Amoco, another large corporation, currently has a method that converts PS waste, including residual food, to an oil that can be re-refined.
The Future
Recycling is a viable alternative to all other means of dealing with consumer plastic waste. In response to the problem of mixed plastic waste, a coding system has been developed and adopted by the plastic industry. The code is a number and letter system. It applies to bottles exceeding 16 ounces and other containers exceeding 8 ounces. The number appears in the 3 bent arrow recycling symbol with the abbreviation of the plastic below the symbol.
Western European companies, especially the German firms Hoechst and Bayer, have entered the recyclable plastic market with success. With a high tech approach, they are devising new methods to separate and handle mixed plastics waste.
A potential use for recycled materials includes plastic lumber. The recycled plastic is mixed with wood fibers and processed into a replacement for lumber. The wood fibers would have become land fill if not reused. The end product is called Biopaste. This is expected to eventually become a multi-million dollar enterprise. Research and development continue to improve this product.
Recycling is a cost effective means of dealing with consumer plastic waste. Research to reduce the cost of recycling needs to continue. Recycling of plastics is not going to reach the level of the recycling programs of paper and some metals until lower cost, automatic methods of recycling are in place. Fortunately, the solutions to these problems are not beyond the scope of our technology or our minds. Below is a chart listing the different types of plastics and their uses before and after they are recycled.
| Resin Code | Resin Name | Common Uses | Examples of Recycled Products |
|---|---|---|---|
 | Polyethylene Terephthalate (PET or PETE) | Soft drink bottles, peanut butter jars, salad dressing bottles, mouth wash jars | Liquid soap bottles, strapping, fiberfill for winter coats, surfboards, paint brushes, fuzz on tennis balls, soft drink bottles, film |
 | High density Polyethylene (HDPE) | Milk, water, and juice containers, grocery bags, toys, liquid detergent bottles | Soft drink based cups, flower pots, drain pipes, signs, stadium seats, trash cans, re-cycling bins, traffic barrier cones, golf bag liners, toys |
 | Polyvinyl Chloride or Vinyl (PVC-V) | Clear food packaging, shampoo bottles | Floor mats, pipes, hoses, mud flaps |
 | Low density Polyethylene (LDPE) | Bread bags, frozen food bags, grocery bags | Garbage can liners, grocery bags, multi purpose bags |
 | Polypropylene (PP) | Ketchup bottles, yogurt containers, margarine, tubs, medicine bottles | Manhole steps, paint buckets, videocassette storage cases, ice scrapers, fast food trays, lawn mower wheels, automobile battery parts. |
 | Polystyrene (PS) | Video cassette cases, compact disk jackets, coffee cups, cutlery, cafeteria trays, grocery store meat trays, fast-food sandwich container | License plate holders, golf course and septic tank drainage systems, desk top accessories, hanging files, food service trays, flower pots, trash cans |